By
Sanofi
Published: Dec. 3, 2015, 10:35 p.m.·
Tags:
Medicines
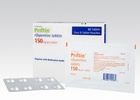
BRIDGEWATER, N.J., Dec. 1, 2015 -- Sanofi announced today the launch of a new 24-count tablet pack of Priftin® (rifapentine) for the treatment of latent tuberculosis infection (LTBI) in patients two years of age and older who are at high risk of progression to tuberculosis (TB) disease. The new 24-count package is tailored for use in the treatment of LTBI and represents one month of Priftin for most patients. The new 24-count packaging features individual tablet printing on the backing foil with individually perforated tablet blisters, and has an extended shelf-life of up to three years.
Read More →
By
Sanofi
Published: Dec. 3, 2014, 8:04 p.m.·
Tags:
Latent TB,
Treatment
Following a priority review, the U.S. Food and Drug Administration has approved Priftin® (rifapentine) in combination with isoniazid (INH) for a new indication for the treatment of latent tuberculosis infection (LTBI) in patients two years of age and older at high risk of progression to tuberculosis (TB) disease. Approved in the United States since 1998, Priftin is an antimycobacterial used in combination with one or more antituberculosis drugs for the treatment of active pulmonary TB caused by Mycobacterium tuberculosis.
Read More →
By
Sanofi
Published: Sept. 24, 2012, 9:21 p.m.·
Tags:
None
Read More →