Data from historic Phase IIb clinical trial for TB vaccine candidate MVA85A published in The Lancet
Vaccine candidate was generally well tolerated, meeting the study’s primary objective of safety; Vaccine candidate did not provide statistically-significant protection in preventing TB disease in infants previously vaccinated with BCG.
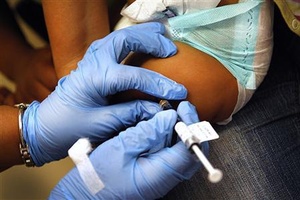
An infant receives an injection as part of a clinical trial of a novel tuberculosis vaccine candidate at SATVI’s clinical trial field site in Worcester
London, UK – Feb. 4, 2013 - Results of a first-of-its-kind clinical trial of a novel TB vaccine candidate show the candidate vaccine was safe and well tolerated, but did not confer efficacy in prevention of TB disease when administered as a boost to Bacille Calmette-Guerin (BCG), the currently used TB vaccine. The clinical trial of the TB vaccine candidate MVA85A was a Phase IIb safety and efficacy trial in 2,797 infants living in the Western Cape province of South Africa. The successfully completed trial, conducted at the highest international standards, was a major global undertaking with the involvement and support of a large number of organizations that joined forces to conduct this historic trial.
The valuable scientific understanding gained from this trial will guide the field moving forward as over a dozen TB vaccine candidates that are currently in clinical trials and a diverse portfolio of preclinical candidtates advance through the R&D process.
>> Read the full press release
>> MVA85A Press Release - French
>> MVA85A Press Release - Flemish
Source: Aeras

